The effect of Allergoff® on house dust mite allergy
The purpose of the study was an attempt to evaluate the efficacy of Der p 1 allergen removal from the environment of a patient allergic to dust with the assessment of the scale of symptoms reduction using Allergoff® Spray, the solution for neutralization of house dust allergens created by ICB Pharma.
The design of these studies has been accepted by the Bioethical Commission of the Silesian Medical University in Katowice.

Research Method
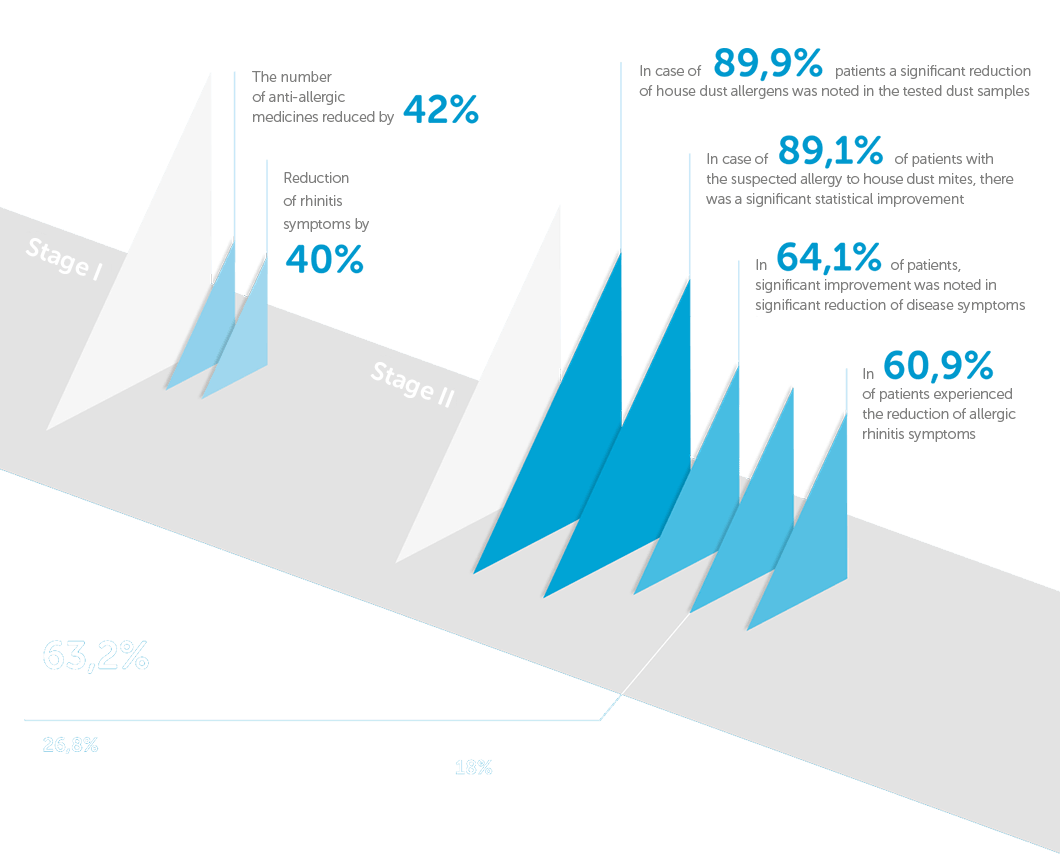
Stage 1
In the first stage of research carried out during the heating season, between December 2016 and April 2017, the study included 42 people, 17 women and 25 men, including 14 people under the age of 18.
Stage 2
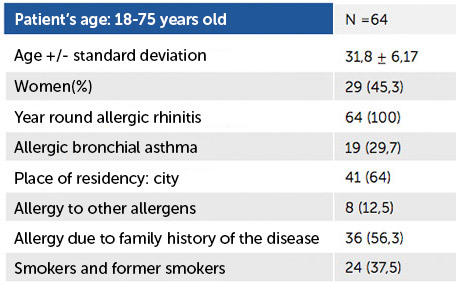
The second stage was carried out on a population of 64 patients between the ages of 18-75 years in the period from November 2017 to April 2018. The characteristics of patients participating in the second stage of the study are shown in Table 1.
Table No. 1. Characteristics of patients participating in the study, stage II
In total, the research group included 106 people, all of whom were residents of the Silesia province.
The criteria for including patients in the study were:
- Symptoms of allergic rhinitis and/or conjunctivitis
- Association of disease symptoms with exposure to house dust based on the patient’s observation
- Anti-allergic treatment
According to the study protocols, the procedure were performed on patients which consisted in one month observation of the symptoms of allergic disease and evaluation of the use of symptomatic drugs before and after the preventive intervention. At the same time, the presence and concentration of the main mite allergen D. pteronyssinus (Der p 1) was monitored in the samples of mattress dust collected and the allergen-specific level of IgE in the blood serum of the examined persons was assessed.
Research procedures
- Clinical evaluation of the patient’s condition before the allergen elimination procedure.
- Dust sampling from the patient’s surrounding in accordance with Annex 1 *.
- The use of Allergoff® in the patient’s environment (bed and its surroundings) in accordance with the manufacturer’s instructions.
- Evaluation of the patient’s clinical condition after one month of the allergen elimination procedure.
- Dust sampling from the patient’s environment one month after Allergoff® use.
Stages of assessing the clinical condition of the patient before and after the allergen elimination procedure:
- Physical examination
- Medical Questionnaire
- Blood collection to assess the level of IgE antibodies for house dust mites
Diagnostic Procedures
1. Evaluation of Der p 1 allergen level in dust samples from the environment of the examined patients.
For the assessment, prepared dust samples from the patient’s environment were taken according to the instructions before and one month after Allergoff® use. The samples were taken from the mattresses and the bed area (vacuuming an area of 1 m2 for 3 minutes). 100 milligram of dust sample was used. The sample was suspended in 1 ml of normal saline in propylene tubes. All samples were frozen in -20°C, until their determination.
After thawing, each sample was treated with 2 ml of buffered 0.05% Tween-20 solution and incubated for 120 minutes at room temperature and then centrifuged for 20 minutes at 3500 RMP at 6°C. Der p 1 allergen was determined in the obtained samples by using monoclonal antibodies in available ELISA kits (Indoor Biotechnologies, Charlottesville, USA). The reference level of Der p1 detection was (0.11 μg / g). The spectrophotometer and the bands of 387 and 390 nm were used for the assessment.
2. Evaluation of the concentration of IgE antibodies against Der p 1 allergen in the serum of the studied patients.
Antibodies were determined by immunoenzymatic method using the Immuno CAP method (ThermoFisher Scientific, Uppsala, Sweden) according to the manufacturer’s instructions. The results were considered positive if the IgE value exceeded 0.35 IU / ml (as indicated by the manufacturer).
In order to reduce the level of exposure to inhalation allergens present in the home dust of patients, the product “Allergoff® allergen-neutralising spray” has been applied to sleeping places including pillows and comforters, the floor area under the sleeping place, carpets and rugs, upholstered furniture, curtains , bedspreads, plush toys, etc. In addition, to neutralize and remove allergens present in fabrics, during the washing of bedding and clothes, a product called “Allergoff® Wash has been used. In the first stage of the study before and after the use of Allergoff®, the level of concentration of mite allergens in the mattress dust tests was evaluated using the “Acarex test” by Allergopharma.
The results
1. Before taking preventive measures by using Allergoff® Spray, it was found that the concentration level of the main mite D. pteronyssinus (Der p 1) allergen in the dust samples from the mattress was high in most cases (the highest value is 0.802 μg / g) or medium from 0.2 – 0.4 μg / g. Only in individual cases the concentration of Der p 1 allergen has been found low as 1 μg / g or at the borderline of detection of 0.009 μg / g.
2. After Allergoff® Spray has been used, the test has been repeated. The results showed the absence of the main Der p 1 allergen in the samples of collected mattress dust, which was analyzed using colored test called “Acarex test” in all patients (100%). On the other hand, based on the detailed allergen detection in the second stage of the study, the average concentration of Der p 1 in 1 gram of the examined dust decreased significantly to 0.176 ± 0.066 μg (Table 2 – the table does not include samples that do not allow to determine the level of allergen in collected dust samples).
3. The demonstrated decrease in the content of the main allergen of D. pteronyssinus mite was correlated with the patients’ subjective assessment during the medical questionnaire. In the first stage, the patients indicated a significant reduction in disease symptoms by an average of 5.4 ± 0.8 (± standard deviation) points on a 10 point scale in relation to the baseline test, where full symptom score was 10 points and 0 indicated complete resolution. This result was statistically significant (p <0.05).
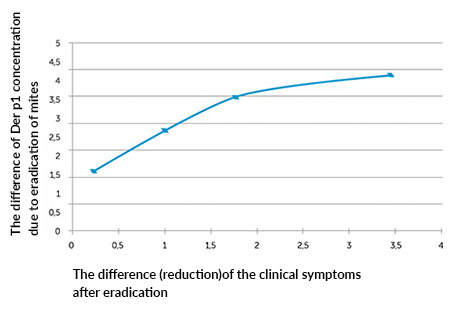
The second stage showed a correlation between decrease in the Der p 1 allergen concentration and the improvement of the patients’ well-being (improvement of the clinical picture expressed by the reduction of nasal symptoms): R = -0.7 for p <0.05 (graph 1).
Graph no.1; Correlation between the degree of reduction of Der p 1 concentration in dust samples after eradication and the degree of improvement of the clinical symptoms.
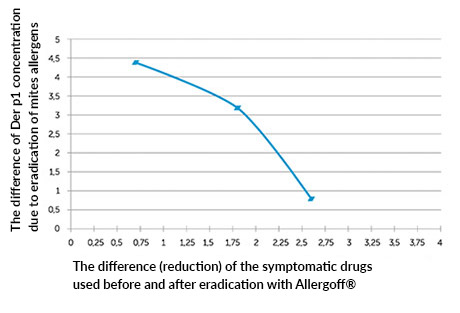
At the same time, the correlation of the decreased concentration of Der p 1 allergen with the reduction of symptomatic drugs used has been demonstrated: R = -0.56 for p <0.05 (graph 2).
Graph no.2; Correlation between the degree of reduction of Der p1 in dust samples after eradication and the degree of used symptomatic medication.
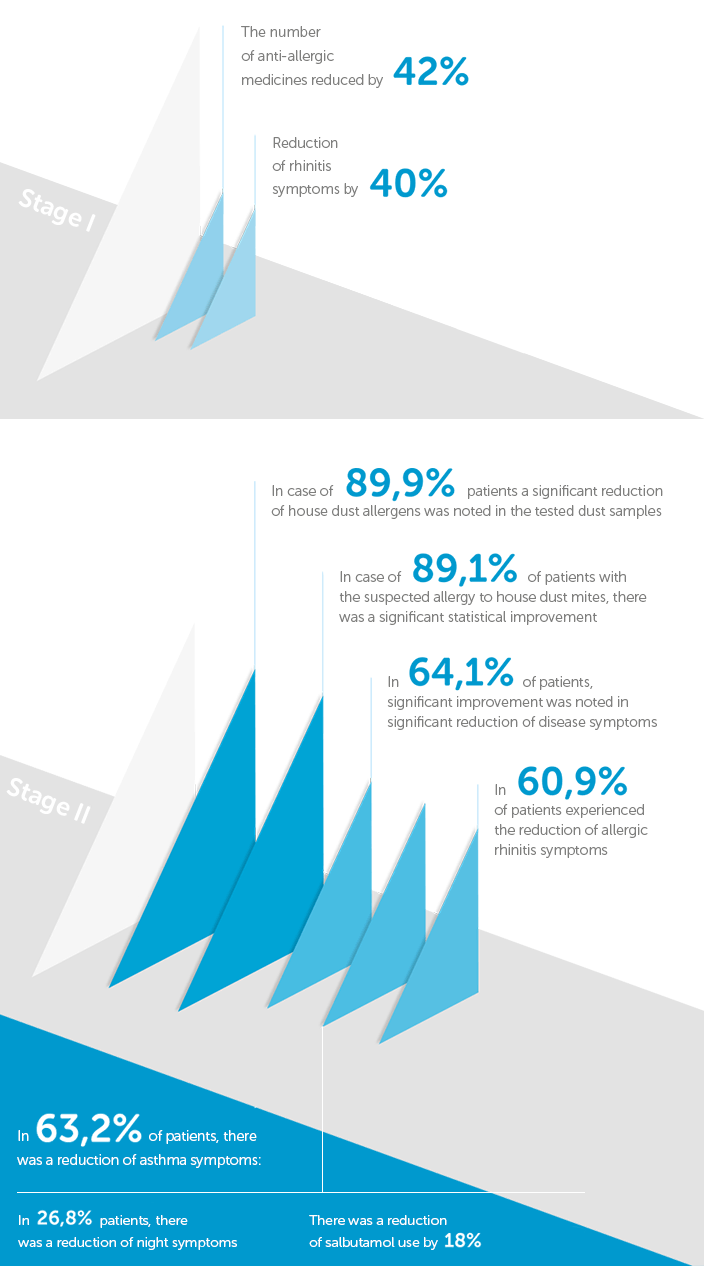
Presentation of some research results
Conclusion
The use of Allergoff® Spray in the patient’s home environment resulted in the decrease in the level of the main mite Dermatophagoides pteronyssinus (Der p 1).
As a result of Allergoff® Spray usage, a significant reduction in clinical symptoms of allergic rhinitis, conjunctivitis and asthma symptoms including night symptoms was observed in the studied patients.
The use of Allergoff® did not trigger any adverse reactions in the studied patients.

The improvement of the clinical picture in the study group occurred already within 30 days after the use of Allergoff® Spray.
The most important goal of asthma treatment is to get control over the disease. That includes normal lung ventilation, a complete absence of night symptoms, reduce activity, the onset of symptoms or the need to use antihistamine medication twice a week at most.
Early results obtained in the group of patients with allergic bronchial asthma shows significant improvement of patients quality of life.
There was a reduction in night symptoms and a reduction in the amount of used medication.
The improvement of the clinical picture in the study group occurred already within 30 days after the use of Allergoff® Spray.
Due to the surprisingly short time in which the quality of patients life was improved and the medication used by the patients was reduced, the study of the effectiveness of prophylactic interventions with the use of Allergoff® Spray will be continued on a larger group of patients.